Verified Science
Brillia Verified Science
We are so glad that you have discovered Brillia! By the time you are done reading, you will have a well-rounded and clear understanding of why Brillia works, how Brillia works, and what makes Brillia a safe and impactful option to both supplements that do not show results and prescription medication that have harmful side effects. We will even walk you through some of the clinical studies that have been done testing Brillia’s claims, side effects (or lack thereof), and longevity of results.
Here at Brillia, we are also parents, and we believe it is important to understand the mechanisms and effects associated with anything we are thinking about putting it in our or our child’s body. That is why we have worked with a team of scientists, hand-in-hand, to put together this comprehensive, yet “non-scientist friendly”, review of everything that you would want to know about Brillia! Let’s start from the very beginning, deciding if Brillia is right for you or your child and their symptoms.
Collapsible content
Who should take Brillia?
We often are asked questions such as, “Does Brillia work for Autism?” or “My child has severe ADHD, can Brillia help?” and to these questions we always have the same answer, “Brillia is designed to target symptoms, not specific disorders” so if any of the following symptoms ring a bell, Brillia can be a great option to try.
So, what symptoms does Brillia target? Brillia is designed to target symptoms of anxiety, stress, hyperactivity, irritability, and lack of focus. Again, the source of these symptoms can be nearly anything, but as long as they are present, Brillia can help knock them out.
So, now that you have had the chance to decide if Brillia is right for you or your child, let’s talk about how Brillia actually works.
What makes Brillia unique?
Brillia is the result of antibody science and homeopathy working hand-in-hand to create a product with the safety associated with homeopathy and the efficacy of active ingredients formulated using science-based methodologies. What does this mean for us? It means Brillia has the efficacy of other prescription medications without the harmful side-effects. Best of both worlds, right?
What is the active Ingredient?
Let’s start off with the active ingredient, registered with the FDA as Lapine S-100B immune globulin. Now we know this name can be intimidating, so we are going to break it down for you. Working backwards, “immune globulin” is just the “sciency” way of saying “antibody”, and don’t worry, we will get into what an antibody actually is in just a second. Next, “S-100B” is the name of the protein the antibody is designed to recognize in the body. Lastly, “Lapine” is just a descriptor of the origin of the antibody, just like the millions of other antibodies used each and every day in laboratories all across the world.
So, what exactly is an antibody? Antibodies are a naturally occurring protein and component of our immune system that are individually programmed to target a very specific protein, in the case of Brillia, the S-100B protein. It is important to understand that antibodies are one of the most specific and targeted molecules in our bodies, resulting in zero off-target effects — meaning that antibodies specifically look for and attach to their target only. This is why Brillia has no harmful side effects, because it only interacts with the S-100B protein. Not only does Brillia have absolutely zero side effects, it also has no contraindications with any other medications or supplements your child may be taking. This is due to Brillia’s extremely high level of target specificity, meaning that Brillia is so well targeted to the S-100B protein, it won’t even think about touching anything else in the body, including any other drugs or supplements.
Now that we know more about the active ingredient, let’s talk about its target, the S-100B protein.
The S-100B protein is a naturally occurring protein and is most prevalent in the brain. It is an important regulator of many processes such as regulating calcium levels and helping neurons communicate, but in our case, we care about how it influences the symptoms we mentioned earlier, such as anxiety and hyperactivity.
Given that S-100B protein influences these symptoms, it is quite intuitive that when the S-100B protein doesn’t do its job properly, these symptoms become more prevalent, and this is exactly what happens in those who suffer from anxiety, hyperactivity, stress and lack of focus.
So, what makes the S-100B protein, for a lack of a better term, mess up? The answer is quite simple, when the S-100B protein is overproduced or overactive, its activity becomes unnecessarily high, making it capable of causing these symptoms.
Why does Brillia work?
Now that we know all about Brillia’s active ingredient and the S-100B protein, let’s talk about why Brillia works!
WATCH: WHY & HOW BRILLIA WORKS
As we mentioned, Brillia’s active ingredient is an antibody designed to target the S-100B protein. We also know that high levels of S-100B result in high levels S-100B activity which result in the symptoms of anxiety and hyperactivity. So, when Brillia builds up in the system, its active ingredient (the S-100B antibody) goes looking for the S-100B proteins to attach to them. When this binding takes place, Brillia stops the S-100B proteins it attaches to from manifesting the symptoms. At the right dosage, Brillia stabilizes the S-100B protein activity back to normal levels. This is why it is very important to choose the right dosage of Brillia to make sure there is enough active ingredient in the system to show results.
To fully understand how the binding of Brillia’s active ingredient to the S-100B protein causes this stabilization (return to normal) of S-100B protein activity levels and thereby a reduction of symptoms of anxiety and hyperactivity, I want to explain how the S-100B protein cause these symptoms, followed by how Brillia inhibits this process.
How does Brillia work?
Before we explain the interactions between Brillia and the S-100B protein, I want to go over the steps any protein in the body goes through in order to cause a change or effect, as this understanding is vital to the mechanism by which Brillia’s active ingredient works.
A protein in the body is just that – a protein. Without binding to its correct target, the protein is completely useless and causes absolutely zero effect in the body. In order for any protein, including the S-100B protein, to take effect and actually have “activity” it needs to bind to its target. In the case of most proteins, including the S-100B protein, the target is an enzyme. Specifically, in the case of the S-100B protein, this enzyme is located in the brain. Enzymes are molecules that help a reaction take place. Once the correct proteins bind to them, they make sure the reaction occurs. Basically, when the S-100B binds to its target, it causes symptoms of anxiety and hyperactivity. In contrast, when it is prevented by binding to its target by the active ingredient in Brillia, these symptoms never have the chance to develop.
So, what does this binding look like? It is actually simpler than you may think! The classic analogy is a lock and key. The protein is the key, and the target is the lock. In order to make sure proteins only activate their correct targets, each protein is programmed to be complementary to its target, exactly like a lock and key! For example, your own house key only opens your door. If you try to put your house key into your neighbor’s door, the door will not open! The same is true for proteins. Each protein has a specific physical shape and will only fit into a target that has the perfect complementary conformation. Only when the protein perfectly matches its target does it begin causing an effect in the body. Proteins can try to fit in to other target molecules, but they will be unable to fit perfectly into the target as their shapes would not be complementary. As a result, no protein activation or effect will result.
Now let’s go back to the context of the S-100B protein and Brillia. With the lock and key analogy in mind, we can imagine that the S-100B protein is a key with a very specific shape and its target is a lock, the perfect complement to the key.
In the diagram below, we have the interactions between the S-100B proteins and their targets without Brillia.

As you can see, the S-100B proteins are all fully capable of finding their target and binding to it, thereby activating the protein. In the analogy of the lock and key, every key fits perfectly into the lock, opening the door to symptoms of anxiety and hyperactivity.
In the following diagram, we see the interactions between the S-100B protein and its target with the administration of Brillia.

The diagram above depicts the S-100B protein having difficulty fitting into its analogous “lock”. Remember when I mentioned the active ingredient in Brillia, the Lapine S-100B immune globulin, attaches to the S-100B protein? This is when that attachment makes a world of a difference. By attaching to the S-100B protein, the overall shape of the S-100B protein has completely changed, similar to sticking a piece of gum on a key. Once that piece of gum (the antibody) has stuck to the key, it won’t fit in to the lock anymore, and the door to all those symptoms of anxiety and hyperactivity remains locked shut. It is important to note that the binding between the antibody and the S-100B protein is transient, meaning it doesn’t last forever. Although antibodies bind very tightly to their target molecule, they will eventually fall off. What does this mean for us as parents? It means that Brillia has absolutely zero long-term implications, the cause of so many of the awful side effects many prescription drugs are riddled with. Considering the fact that the binding between Brillia and the S-100B protein is transient, we hope you realize the importance of giving your child their doses both on time and regularly, as Brillia’s efficacy depends on having a stable level of active antibodies in the system at all times.
Are there studies to show that Brillia works?
Now that you understand how Brillia works, I am going to prove to you that it does work. Clinical studies are often extremely complicated to read, let alone understand. That is why we took it upon ourselves to do all the hard work for you and hand-pick the most important parts of some of the clinical studies that have been done on Brillia’s efficacy.
Before we get into any data, I want to describe a key component of a clinical trial. There are typically two groups to a clinical trial, an experimental group and a control group. The control group is sometimes called the “placebo” group. The only difference between those assigned to a control/placebo group or an experimental group is the administration of the product you are testing, in this case Brillia. Those in the experimental group were dosed with Brillia while those in the control/placebo group were given something else with no active ingredient.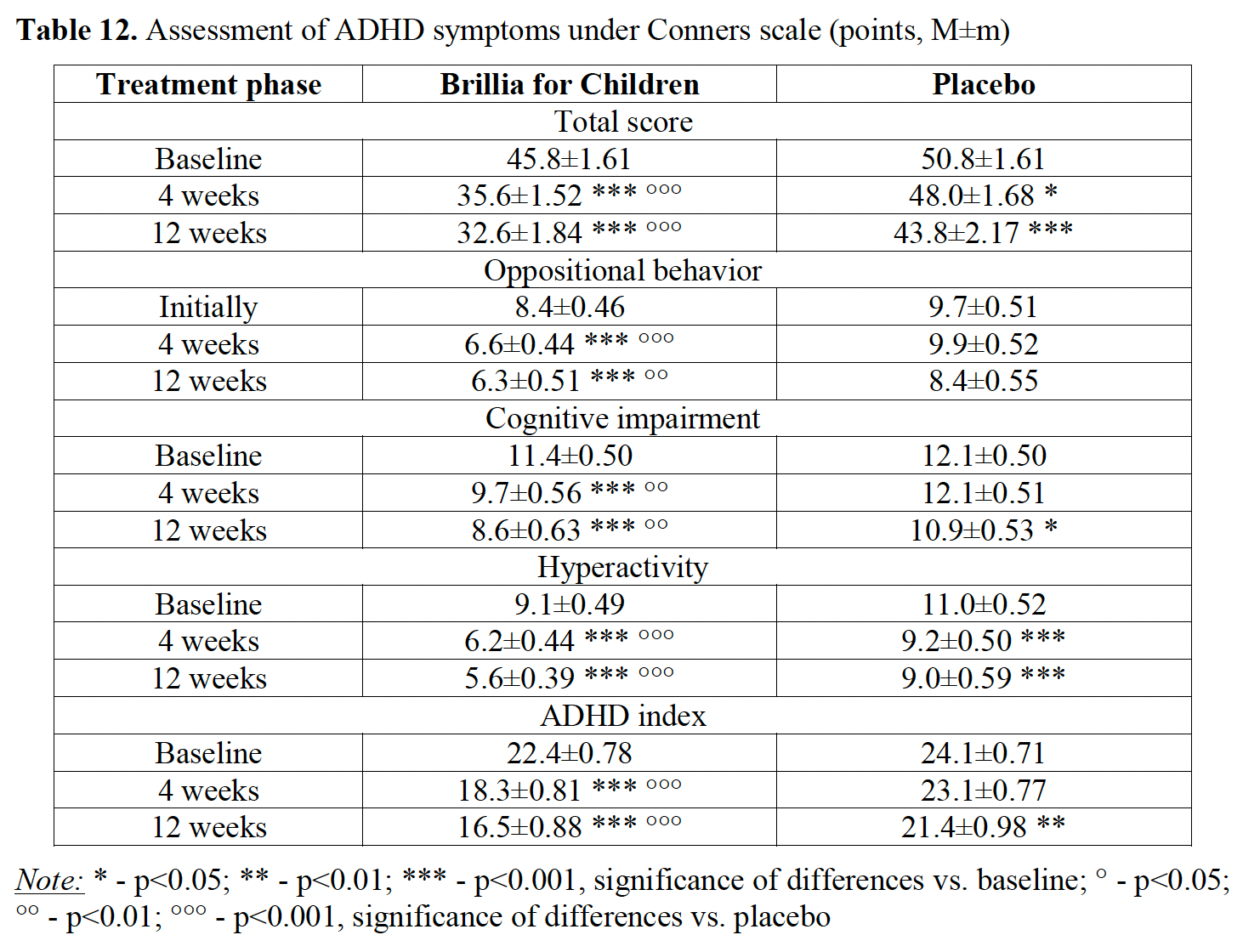
Above is a table with various “scores” on the Conners Scale (a scale psychiatrists use to quantify the severity of various behavior disorders) for a variety of symptoms such as hyperactivity, anxiety, etc. with numbers corresponding to the severity of ADHD. The higher the score, the more severe the symptoms. In addition, there is a total score that is based on all the other values. This study was also done over a period of 12 weeks. By comparing the differences in “scores” over the 12 weeks between the placebo and Brillia group, we can evaluate how well Brillia works.
The simplest values to understand are those corresponding to the “Total Score”. Initially, the Brillia group had a score of 45.8 while the placebo group had a score of 50.8. After the 12-week period, the Brillia group had a score of 32.6 while the placebo group had a score of 43.8. To properly evaluate these results, we have to look at how much each score went down after the 12-week period. In the Brillia group, the score went down from 45.8 to 32.6. This means the score went down by 13.2 points. In contrast, the placebo group (those not given Brillia), only decreased from 50.8 to 43.8, a small 7-point decrease. Therefore, we can conclude that those given Brillia had a much more significant decrease in severity of their symptoms, supporting Brillia’s efficacy.
Above is another table very similar to the previous one, except this time the scores are assigned by the parents of the children in either the Brillia or placebo group rather than using the Conners Scale.
Before the trial began, the Brillia group had a score of 12.7 points while the placebo group had a score of 13.0 points. After the 12-week trial, those that were given Brillia decreased to a score of 6.8 points while those given the placebo only decreased to 10.0 points. This means that those who took Brillia demonstrated a 5.9-point drop in ADHD severity while those in the placebo group only demonstrated a 3.0-point drop in ADHD severity. Furthermore, the severity of ADHD in the Brillia group was nearly cut in half (12.7 to 6.8) while there was very little change in the placebo group (13.0 to 10.0). Once again, this data demonstrates Brillia’s ability to significantly alter severity of symptoms, especially when compared to the placebo/control group.
The following graph demonstrates the proportion of those in each group with severe, moderate, or mild anxiety over a time period of 12 weeks and between Brillia and placebo groups. Measurements of severity are taken every four weeks. On the x-axis, the one that runs horizontally at the bottom, the groups are notated – those administered Brillia or and those who are given a placebo.
What information can we extract from this graph? We see that before any administration of Brillia or the placebo, the groups have similar distributions of anxiety level. The difference between the percentages with moderate anxiety between the groups is only 4, and the difference between the percentages with sever anxiety is only 6. After the full 12 weeks, we see that there is a larger difference between the percentages between the placebo and Brillia groups. The placebo group has 35% severe anxiety and 65% moderate anxiety with 0% mild anxiety. In contrast, the Brillia group has only 20% severe anxiety (15% less than the placebo group) and 78% moderate anxiety. In addition, there is even 2% that now have only mild anxiety in the Brillia group while the placebo group has 0% with mild anxiety.
This shows that over the course of 12 weeks, Brillia had a significantly better effect on the severity of anxiety over those that did not take Brillia, therefore proving Brillia’s efficacy.
Does Brillia’s effect diminish over time?
Brillia is designed to be administered in very low doses, up to three times a day. This way, the concentration of Brillia in the body is maintained so it can properly act upon the overactive S-100B proteins and alleviate symptoms such as anxiety and hyperactivity.
Unlike many prescription drugs, the body does not develop any tolerance to Brillia, so dosage never needs to be increased and Brillia’s effects never diminish, unless of course the severity of your child’s symptoms call for a higher dosage of Brillia than you originally decided to give your child.

Here we have a scatter plot with a single data point plotted every four weeks up to a total of 12 weeks. On the x-axis, we have each time point after Brillia was first administered. On the y-axis, we have the severity of symptoms. We see that even after 12 weeks, the severity of symptoms continues to decrease. This shows that Brillia’s efficacy does not diminish over time, nor does the body develop any sort of tolerance for Brillia refraining from the need to ever increase dosage.
Is Brillia safe?
The safety of the active ingredient is tested by analyzing the number of “adverse events” that occur between the experimental and placebo/control group throughout the course of a clinical trial. Adverse events are considered any changes to the health of the individual, such as cold symptoms, an allergic reaction, redness, etc., during the course of the trial. In order to determine if the adverse events were due to the administration of the active ingredient, the number and type of adverse events between the experimental and placebo groups are compared. Unless the experimental group (taking Brillia) had significantly more adverse events, the active ingredient is considered just as safe as an individual that wasn’t administered the active ingredient at all, such as those in the placebo group (not taking Brillia).
Table 18. AEs in placebo and Brillia for Children groups

Here we have a table demonstrating the number and type of adverse events that occurred throughout the course of the trial. The table is split into two groups, adverse events that occurred to those in the experimental group (taking Brillia) and those in the placebo group (not taking Brillia).
In total, there were 14 adverse events in the Brillia group and 12 in the placebo group. After sufficient statistical analysis, there is no significant difference between the number of adverse effects between the two groups. Therefore, Brillia is determined to be completely safe.
Can we answer any other questions?
Contact our Customer Care below, or give us a call at 1 888 411 6952

